Again consider the reaction in equation 3 above. First convert this volume into mass using density (g/mL), then convert grams to moles using the molecular weight. Again, include units and set up your calculation so that milliliters and grams cancel in the calculation leaving an answer that has units of moles. To calculate molarity, divide the number of moles of solute by the volume of the solution in liters.
Once you have the molar mass, multiply the number of grams of solute by 1 over the molar mass to convert the grams into moles. Finally, divide the number of moles by the volume of the solution to get the molarity. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent. This is because when you add a substance, perhaps a salt, to some volume of water, the volume of the resulting solution will be different than the original volume in some unpredictable way. To get around this problem chemists commonly make up their solutions in volumetric flasks.
These are flasks that have a long neck with an etched line indicating the volume. The solute is added to the flask first and then water is added until the solution reaches the mark. The flasks have very good calibration so volumes are commonly known to at least four significant figures. To calculate the molarity of a solution, the number of moles of solute must be divided by the total liters of solution produced. An aqueous solution consists of at least two components, the solvent and the solute . Usually one wants to keep track of the amount of the solute dissolved in the solution.
One could do by keeping track of the concentration by determining the mass of each component, but it is usually easier to measure liquids by volume instead of mass. To do this measure called molarity is commonly used. Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. The units of molar concentration are moles per cubic decimeter.
They are noted as mol/dm³ as well as M (pronounced "molar"). The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute, e.g., the concentration of hydroxide anions can be written as [OH⁻]. In many older books or articles, you can find different units of molar solutions – moles per liter (mol/l). Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values.
Molality is an intensive property of solutions, and it is calculated as the moles of a solute divided by the kilograms of the solvent. Unlike molarity, which depends on the volume of the solution, molality depends only on the mass of the solvent. Since volume is subject to variation due to temperature and pressure, molarity also varies by temperature and pressure.
In some cases, using weight is an advantage because mass does not vary with ambient conditions. For example, molality is used when working with a range of temperatures. To get to moles, use the equation and the molar ratios shown. To get to volume, use the molar volume of gas constants.
To get to mass, use the atomic/molecular masses shown in the periodic table. We can then convert these amounts in moles to mass, volume etc. as required. One mole of an ideal gas has a volume of 22.4 L at standard temperature and pressure. Is the volume occupied by one mole of a chemical element or a chemical compound. It can be calculated by dividing the molar mass by mass density (ρ).
Molar gas volume is one mole of any gas at a specific temperature and pressure has a fixed volume. Thus, we can rearrange terms in the ideal gas equation and write them like this . The behavior of gases can be described by several laws based on experimental observations of their properties. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law).
The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law). Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law). In chemistry and physics a mole describes an amount of a substance in grams equal to its atomic mass.
For example, one mole of aluminum has a mass of 13 grams since it has an atomic mass of 13. Also, one mole of a substance contains Avogadro's number of atoms, namely 6.02 times 10 to the power 23. The molarity, or concentration of a solution, equals the number of moles in the solution divided by its volume. Conversion between moles, molarity and volume is performed frequently in science problems. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law.
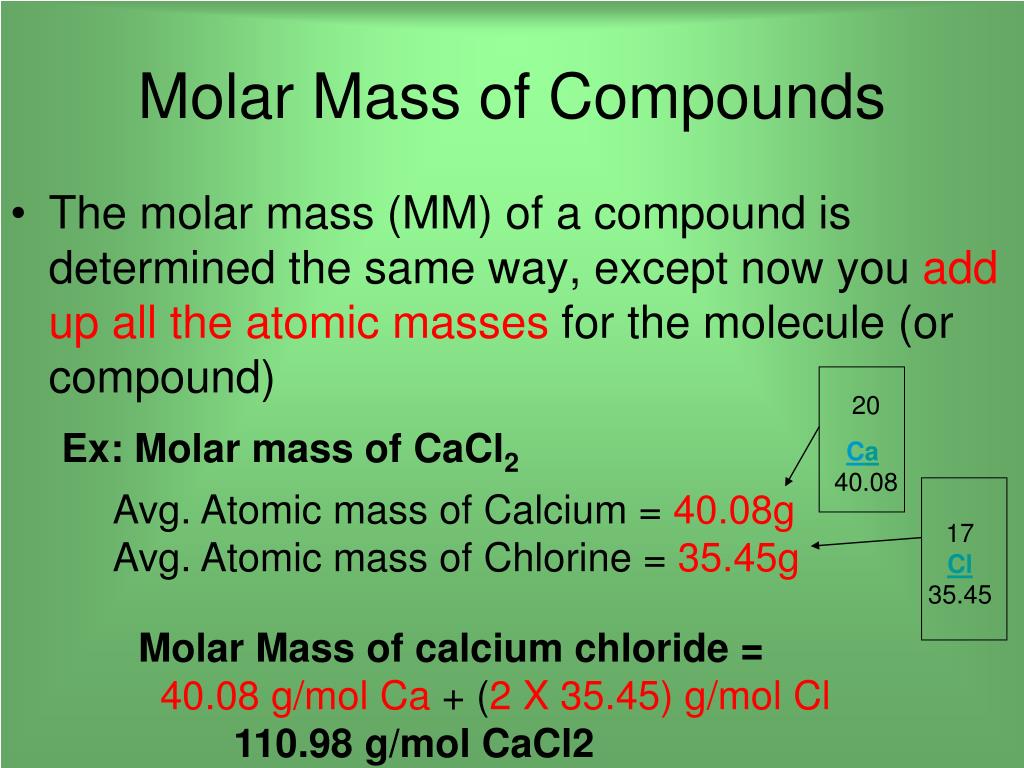
You can calculate molar mass by finding the number of atoms in the molecular formula, then adding the atomic mass of each element together to find the molecular weight. The atomic mass can be found on the periodic table. The ideal gas law formula states that pressure multiplied by volume is equal to moles times the universal gas constant times temperature. The concentration of a substance is the quantity of solute present in a given quantity of solution. Concentrations are usually expressed as molarity, the number of moles of solute in 1 L of solution. We then convert the number of moles of solute to the corresponding mass of solute needed.
Figure 4.6 "Preparation of a Solution of Known Concentration Using a Solid Solute" illustrates this procedure for a solution of cobalt chloride dihydrate in ethanol. Note that the volume of the solvent is not specified. Because the solute occupies space in the solution, the volume of the solvent needed is almost always less than the desired volume of solution.
For example, if the desired volume were 1.00 L, it would be incorrect to add 1.00 L of water to 342 g of sucrose because that would produce more than 1.00 L of solution. As shown in Figure 4.7 "Preparation of 250 mL of a Solution of (NH", for some substances this effect can be significant, especially for concentrated solutions. Avogadro was an Italian Physicist who first described the Avogadro constant as a hypothesis in 1811. He was trying to understand why in chemical reactions involving gases the observation that equal volumes of different gases had the same number of moles. This was found true even when the masses were very different. The idea that a mole of any substance has exactly the same number of atoms no matter what the substance is made of was explained by Avogadro and his name has stuck to his number ever since.
Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute per kilogram of solvent . • To convert volume to moles, first convert to mass using density, then convert to moles using molecular weight. Again, be sure to include all units in your calculations. You can get your moles by taking the molar mass of each of the elements in the solute and adding them together.
Do it; the answer is in moles because the grams cancelled out.Then, go ahead and do your formula. The molar volume of gas at STP, standard temperature and pressure (0°C or 273K, 100 kPa pressure) is 22.4 litres per mole (22.4 L/mol). In other words, one mole of atoms of a pure ideal gas at 0°C will fill 22.4 litres of space.
The molar volume of gas at room temperature (25°C, 298K) and pressure is 24 litres per mole (24 L/mol). Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures.
Two solutions that have the same molarity will have the same number of molecules of the chemical per liter but are likely to contain differing masses of that chemical per liter to achieve this. Provided some additional information is known, one value can be deduced from the other using the equations below. Put a septillion atoms of iron on the scale and we'd have 93 grams of iron! We have an amount that is easily seen and easily weighed. So why isn't a septillion our standard amount of substance?
Well it could have been, but since the amount we pick is somewhat arbitrary, why not pick the number that would make the mass on the scale MATCH the atomic weight of the element? Totally doable and that is exactly how the mole was born and to be specific - how that 6.022 × 1023 number (Avogodro's number) was decided on back in the day. Go get yourself Avogadro's number of atoms of any element and weight it.
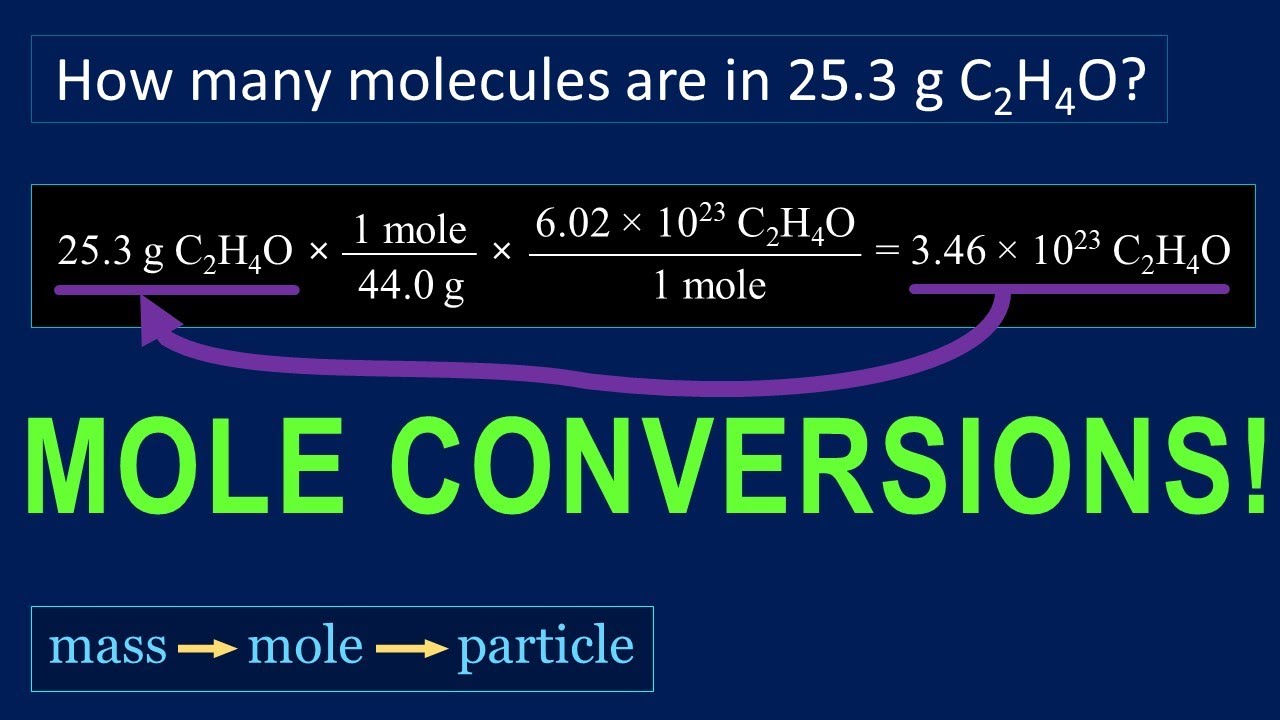
You'll get exactly the atomic weight value in grams. This works for molecules too - you'll get the molecular weight in grams when you have a mole's worth on the scale. The molar concentration unit [mol/ L ] is a conventionally widely used as concentration method. It is the number of moles of target substance dissolved in 1 liter of solution. Molecules are composed of several atoms, for example a carbon dioxide molecule is made up of 1 carbon atom and 2 oxygen atoms.
The molecular weight (or molecular mass or relative molecular mass ) is the sum of the atomic weights of all the atoms in the molecule. Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with 22.4 L at STP, and use molar mass and mole ratios to figure out how many products or reactants we have. So you are not confused with similar chemical terms, keep in mind that molarity means exactly the same as molar concentration .
Molarity expresses the concentration of a solution. It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!). • Mass can be converted to moles using molecular weight. Be sure to include all units in your calculations.
By insuring that the mass units cancel in the calculation you can be sure you have the calculation setup properly. The relationship among the moles of a gas, the volume of the gas in liters, and the molar volume is similar to the mass-to-mass formula. The process for converting the volume of ideal gas to moles is a little different than mass. You'll still use a formula, but you do not need the molar mass of the substance for the conversion. Of course, if you don't have the molar mass handy and want to skip this step, you can use our molar mass calculator to easily calculate the molecular weight of a substance. The molarity calculator calculates the mass of compound required to achieve a specific molar concentration and volume.
To dilute a solution of known molarity, please use the Solution Dilution Calculator. To dilute a solution of concentrated acid or base of known w/w% strength, please use the Acid & Base Molarity Calculator. To calculate molarity, you can start with moles and volume, mass and volume, or moles and milliliters.
Plugging these variables into the basic formula for calculating molarity will give you the correct answer. An alternative way to define the concentration of a solution is molality, abbreviated m. Molality is defined as the number of moles of solute in 1 kg of solvent.
Would you expect a 1 M solution of sucrose to be more or less concentrated than a 1 m solution of sucrose? Concentrations are often reported on a mass-to-mass (m/m) basis or on a mass-to-volume (m/v) basis, particularly in clinical laboratories and engineering applications. Each measurement can be expressed as a percentage by multiplying the ratio by 100; the result is reported as percent m/m or percent m/v. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter.
These concentrations and their units are summarized in Table 4.1 "Common Units of Concentration". The interest stems from that accurate measurements of the unit cell volume, atomic weight and mass density of a pure crystalline solid provide a direct determination of the Avogadro constant. Where Z is the gas compressibility factor, which is a useful thermodynamic property for modifying the ideal gas law to account for behavior of real gases. The above equation is basically a simple equation of state . Molarity is not the same as concentration, although they are very similar. Concentration is a measure of how many moles of a substance are dissolved in an amount of liquid, and can have any volume units.
Molarity is a type of concentration, specifically moles per liter of solution. Chemists use many different units for describing concentration. However, the term molarity, also known as molar concentration, is the most common way of expressing the concentration. When the reactants are expressed in mole units, it allows them to be written with integers in chemical reactions. First, let's take a closer look at what is the mole, so we can move on later to find what is molarity.
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration . You can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you with the molarity definition and the molarity formula. It is easy to calculate molality if we know the mass of solute and solvent in a solution. Molality is an intensive property, and is therefore independent of the amount being measured. This is true for all homogeneous solution concentrations, regardless of if we examine a 1.0 L or 10.0 L sample of the same solution.
The concentration, or molality, remains constant. Now that you're armed with the molar mass of the substance, you can calculate the quantity in moles using the total mass in grams. Now that you have the molar mass of the solute, you need to multiply the number of grams of solute in the solution by a conversion factor of 1 mole over the formula weight of the solute. This will give you the number of moles of the solute for this equation. Methods to calculate number of moles of chemicals in reactions using mass, moles and volume of gas. The most common molar volume is the molar volume of an ideal gas at standard temperature and pressure (273 K and 1.00 atm).
























No comments:
Post a Comment
Note: Only a member of this blog may post a comment.